Home |
|
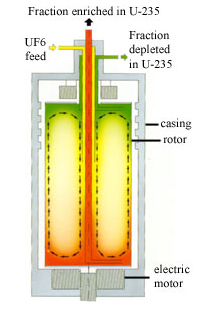
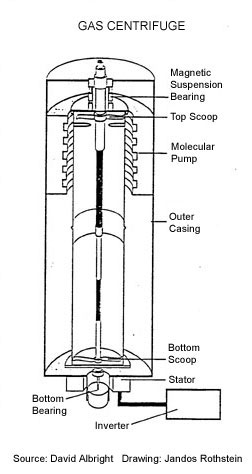
In nature, uranium contains less than 1 percent of the fissile isotope uranium 235 (U-235). A nuclear explosive needs uranium enriched to at least 20 percent U-235. Ideally greater than 90% U-235 is used. In order to increase the percentage of U-235 in relation to the more prevalent U-238 the uranium must be processed, or “enriched.” One technique to enrich uranium uses gas centrifuges. A gas centrifuge (diagrams below) comprises an evacuated casing containing a cylindrical rotor which rotates at high speed in an almost friction-free environment. The uranium is fed into the rotor as gaseous uranium hexafluoride (UF6)2 which also rotates. The centrifugal forces push the heavier uranium 238 (U-238)
closer to the wall of the rotor than the lighter U-235. The gas
closer to the wall becomes depleted in U-235 whereas the gas nearer
the rotor axis is enriched in U-235. The enrichment effect of a single centrifuge is small, so they are linked together by pipes into cascades. Passing through the successive centrifuges of a cascade, the U-235 is gradually enriched to the required level. For civil applications, natural uranium containing about 0.7 percent U-235 is enriched to about 3-5 percent U-235 and the depleted uranium contains typically about 0.2-0.3 percent U-235. For military applications, highly enriched uranium (HEU) containing greater than 20 percent U-235 is usually produced. Once started, a modern centrifuge runs for more than 10 years with no maintenance. An advantage of the centrifuge process is its low energy consumption.
1Source: www.urenco.com 2 Uranium hexafluoride (UF6) is a solid white material at room temperature, which evaporates into gaseous material at elevated temperatures.
|
|||

|
|
 |